Major headlines were made when it was announced that
AstraZeneca Settles Case Over Marketing of a Drug For $520 Million. This is not the first and will not likely be the last off-label settlement by a drug company. The charges from the Justice Department , which the company denies, claim that Astra
Zeneca (from
Med Page Today) paid doctors for "ghostwritten journal articles that the named authors did not write and reported studies they did not conduct" and "to give promotional lectures to other
health care professionals about unapproved and unaccepted uses" for their drug
Seroquel (
quetiapine) which is used to treat bipolar disorder. Though ghost written articles may not be the best way to promote your product, they are not illegal. In addition, paying physicians to speak about a product is an FDA approved way for drug companies to discuss their products with practicing providers. These activities were not the problem, its that (the Justice Department claims) they were off-label, not on label promotions.
I blogged about what is meant by labeling a while ago in a post called
An Indication For Change. Briefly, a physician can prescribe any FDA approved drug for any reason they want. However, a drug company can only promote (sell) that drug for what the FDA says it can be used for. A great example would be
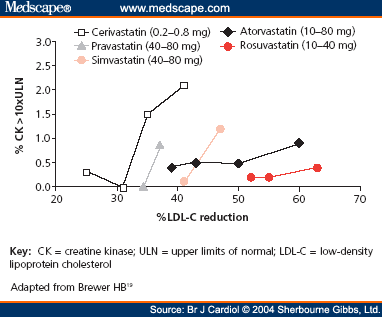
Crestor. Back in November of 2008, I blogged about the results of the Jupiter trial (see
Jupiter is Out, and the News is Good! ), which showed that in patients who had relatively normal cholesterol levels but high
CRP levels, 20mg of
Crestor reduced the risk of heart-related death, heart attacks and other serious cardiac problems by 44%. This was a large, randomized clinical trial with important information and the first real trial showing major benefit when a
statin was used as primary prevention for heart disease in people with normal cholesterol levels. Despite the exciting news, the
Crestor drug reps couldn't talk about it, even though the study was published, and the results were headlines in every major media outlet. This would have been considered off-label promotion. It wasn't until February 2010, when
Crestor was approved for primary prevention of cardiovascular disease by the FDA.
I will admit that I do not write
Seroquel, and told many of the AZ drug reps who tried to get me to use this drug, that
Seroquel was not really a primary care drug, and they would be better off spending their time speaking with the psychiatrists. However, though I am certain one or two rogue drug reps probably bent the rules here and there, given the previous large settlements by other drug companies, I find it very hard to believe that AZ systematically promoted
Seroquel for off-label use. As stated AZ denies these claims.
If this is indeed the case, then I believe AZ should fight the claims by the Justice Department, rather than just pay a fine. If you are really innocent, then you will try to prove your innocence at any cost.
As a physician, I understand what settling a case means. The vast majority of malpractice claims are settled. This is because doctors get nervous when their fate is left to a jury to decide between the rich doctor and the injured patient. For physicians, especially given the cost of litigation, it often makes more sense to settle, admitting no wrong, and get on with your practice then to prove your innocence. However, with
pharma's deep pockets, cost should not be an issue. Every time a drug company settles a claim for off label promotion, it is saying to patients and physicians "we are not to be trusted." As part of their settlement, AZ will likely have to send "Dear Doctor" letters to all physicians essentially admitting guilt.
Interestingly, The Wall Street Journal is reporting in a post
Whistleblower Twice Over: First Lilly, Now AstraZeneca, that the same person who reported AZ to the government (and will pocket $45 million) previously blew the whistle on Eli Lilly who settled a $1.4 billion for off label promotion (he pocketed up to $100 million for that). The fact that the same person blew the whistle on two companies for the same charge (in my mind) calls the entire off-label promotion scheme into question.
This will not be the last big settlement for off-label promotion. The government makes big bucks on these cases, and the drug companies just write a check when fined. If
pharma really want to show physicians, patients and the public that it is just trying to make useful medications to help people and not trying to make a profit any way they can, they should fight these claims as hard as possible.